Weekly Checkup
July 26, 2024
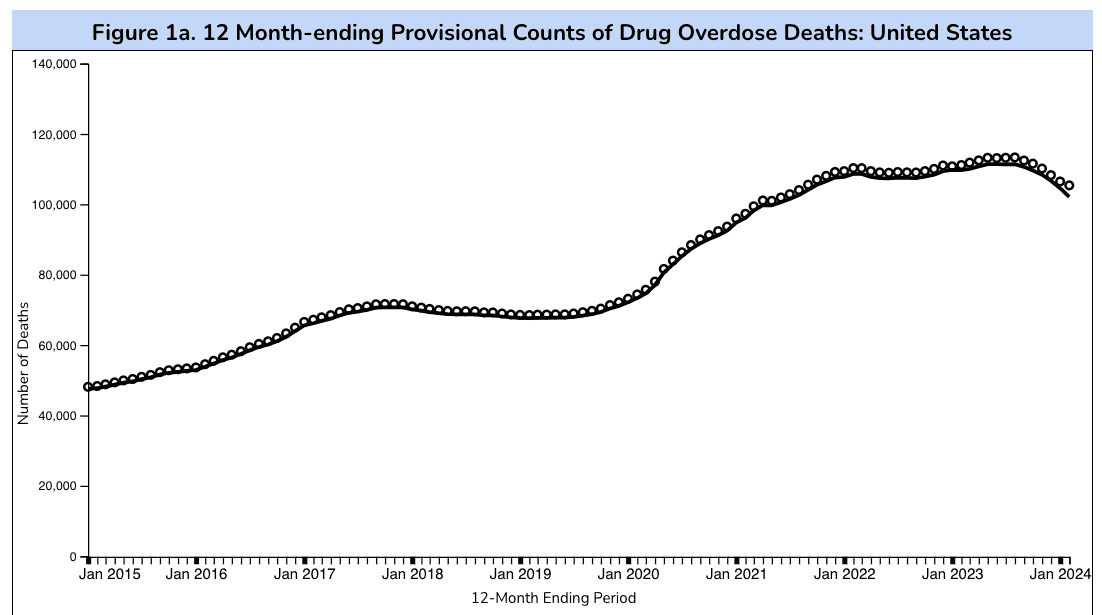
A Few Ways to Combat Opioid Deaths
In early July, the Centers for Disease Control and Prevention (CDC) published updated provisional data (for the 12 months ending in February 2024) detailing the percentage change in reported total drug overdose deaths. This new data shows a noticeable decrease of 8.4 percent in total reported overdose deaths compared to the peak count of 111,435 total in June of 2023. While this figure does highlight some improvement in the country’s decades-long struggle to curb U.S. overdose deaths, this decrease represents only a small improvement in light of the roughly 240 percent growth in reported overdose deaths the United States has experienced since January 2015. Let’s discuss two of the most effective initiatives for curbing overdose deaths, as well as challenges in their current implementation.
Source: CDC.gov
As highlighted in a previous Weekly Checkup, U.S.-based opioid overdose deaths (OOD) are categorized into four waves of opioid use. Beginning in the early ’90s, the first two waves of opioid use caused an exponential growth of OODs. Spurred by high opioid prescription rates and black tar heroin, the United States saw a 540 percent increase in OODs, peaking at 15,469 deaths in 2016. Later, fueled by fentanyl, synthetic opioids surpassed heroin and prescription drugs as the most used opioids, signaling the beginning of the third wave. Moving to current day, many experts believe the United States entered a fourth wave of OODs in early 2023. Marked by a drastic increase in the consumption of fentanyl-cut methamphetamine and cocaine, U.S. OOD soared, peaking at 173,751 reported opioid deaths in June of 2023. Lawmakers have a few cost-effective strategies for reducing the country’s opioid crisis: improving naloxone access and enhancing prescription drug monitoring programs (PDMPs).
As a brief refresher, naloxone (brand name Narcan) is an emergency medication used to treat and rapidly reverse opioid overdose, while PDMPs are state-based electronic databases that track and record any controlled substance prescriptions a patient receives. As a study conducted by the Stanford-Lancet Commission contends, the most efficient intervention for reducing OODs is a combined intervention of a “30 percent increase in naloxone expansion combined with either reductions in all prescribing or with PMPs [PDMPs],” which leads to “approximately 0.1% gains in life years and QALYs [quality-of-life years] and approximately 27% reductions in opioid deaths over five and ten years.” But there have been significant issues in implementing this strategy.
In March of 2023, the U.S. Food and Drug Administration (FDA) approved Narcan as an over-the-counter (OTC) treatment for opioid overdose. While removing the prescription barrier for Narcan represents a significant step toward improving general naloxone access, the drug’s high out-of-pocket cost of $45 per dose may present a substantial hurdle to access, especially for at-risk populations that would benefit most from a reduced burden of access. Additionally, some overdose cases can also require multiple Narcan administrations, further exacerbating costs.
Following the FDA’s reclassification of Narcan from a prescription to an OTC drug, Medicare enrollees across the country lost coverage of Narcan because Medicare does not cover OTC drugs. In response, states began covering OTC Narcan under their state Medicaid programs – but required that beneficiaries get a prescription for the drug to receive coverage. This presents another issue, as requiring a prescription often reduces uptake, leaving those in need of Narcan coverage and access in a challenging position: Either they pay the OTC cost of Narcan (which many won’t), or they get a prescription for covered Narcan (which many won’t).
PDMPs, the other arm of the Stanford-Lancet Commission’s strategy, also have their share of issues. As of May 2024, every state has implemented some form of a PDMP, but four states, Oregon, Montana, Nebraska, and Missouri, have yet to pass PDMP-use mandates. These mandates effectively require providers to consult a PDMP database before writing a controlled substance prescription. As demonstrated by a study published in Health Affairs, not only are PDMP mandates associated with significant reductions in opioid-related emergency department visits and inpatient stays, but full implementation of these mandates across the United States could save Medicaid roughly $155 million in annual spending (based on 2019 Medicaid prescription data).
While it is commendable that the U.S. experienced its first noticeable drop in OOD since 2020, lawmakers should remain aware that this decrease is minor compared to the drastic growth in total OODs the country has experienced over the past several decades. To continue building on this growing reduction in OOD, Congress may consider improving Narcan access for at-risk populations and incentivizing hold-out states to fully institute PDMP mandates.
COVID-19 Variant Prevalence in the U.S. Summer Surge
Parth Dahima, Health Care Data Analyst
The “summer surge” of COVID-19 cases across the United States was predicted by the Federal Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee. Fueling this surge are the latest variants of COVID-19, which account for more than half of all current cases. These new variants – a part of the newly designated FLiRT family – are all descendants of the Omicron variant JN.1 but were initially unaccounted for in the FDA’s initial approach in early June.
The chart below displays projected estimates of variant proportions in the United States. Most notable is the prevalence of the KP.3 variant, which accounts for nearly a third of COVID-19 contractions. The second and third leading variants (KP. 3.1.1 and KP. 2.3) account for 17.7 percent and 12.8 percent, respectively. The LB.1 variant, known for its comparatively high transmission rate, sits fourth at 10.5 percent.
With the emergence of KP.2 fueling the increase in cases, the FDA formally requested vaccine manufactures to target the KP. 2 offshoots in their next round of vaccines. Both Moderna and Pfizer indicated compliance with the FDA’s request while Novavax stated that its planned JN.1 vaccine neutralizes multiple variant strains, including KP. 2.
Sources:
https://covid.cdc.gov/covid-data-tracker/#variant-proportionsnt Proportions